Reactions of Diazonium Salts: Sandmeyer and Related Reactions
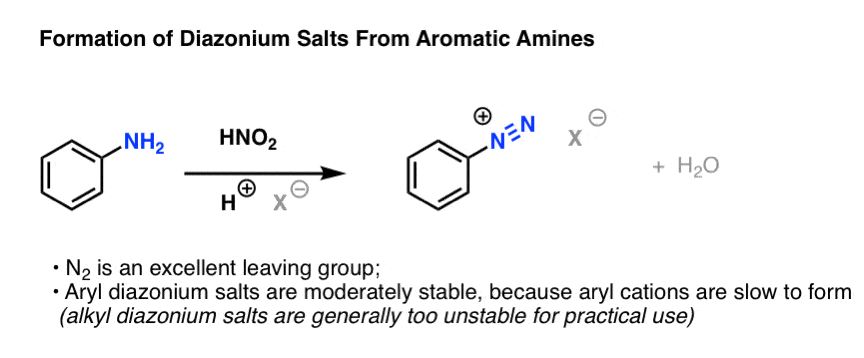
Formation of Diazonium Salts From Aromatic Amines
Today let’s talk about a set of reactions of aromatic amines, that variously are catalogued under “amines” and “aromatic compounds”, depending on the textbook.
It involves converting an aromatic amine (NH2) into a tremendously good leaving group (N2) which can then be replaced by various nucleophiles. However, N2 is such a good leaving group that the method only works well for aromatic amines; alkyl (“aliphatic”) amines tend to lose N2 too rapidly, making the method much less useful in that case.
Here’s the process. Treatment of an aromatic amine with nitrous acid (or sodium nitrite, which is converted to nitrous acid in the presence of acid) in the presence of a strong acid like HCl results in the loss of H2O and the formation of a new N-N triple bond. The resulting species is called a “diazonium ion”:
(How does it work? We’ll go through the mechanism at the bottom of the post).
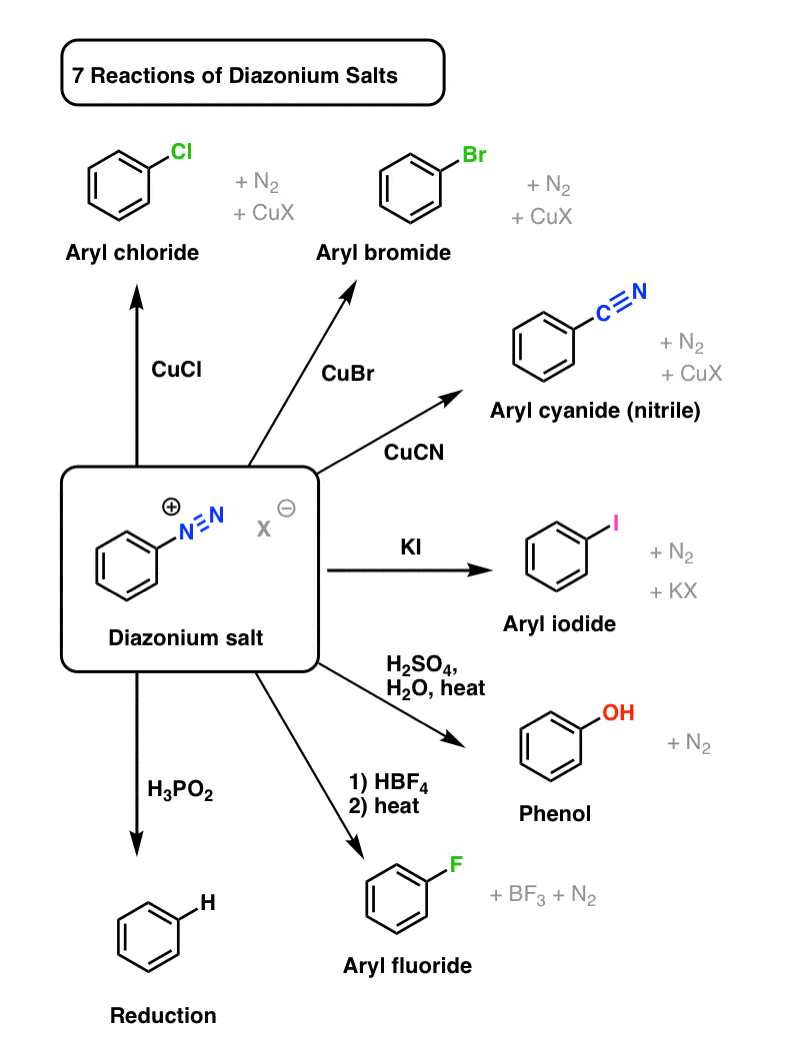
Reactions of Diazonium Salts
So why does it matter?
It matters because the resulting diazonium salts can be transformed into all kinds of useful functional groups. Rather than describe everything in words, first let’s just show 7 examples with a diagram:
Any process featuring a single starting material that can be transformed into seven different potential products can be reasonably described as “versatile”.
These reactions can be roughly divided into two categories: Sandmeyer reactions, and everything else.
Sandmeyer Reactions
One way to transform diazonium salts is by treating them with various compounds of copper. These are known as Sandmeyer reactions, after Traugott Sandmeyer who first discovered the reaction in 1884 (with copper acetylide!).
Three key examples are:
- CuCl transforms aryl diazonium salts into aryl chlorides
- CuBr transforms aryl diazonium salts into aryl bromides
- CuCN transforms aryl diazonium salts into aryl cyanides (nitriles).
The mechanism, which you can read about elsewhere, likely proceeds through an aryl radical, which is oxidized to an aryl cation and then attacked by a nucleophile.
Other Reactions
Copper isn’t necessary for substitution to occur if a strong enough nucleophile is present, or if the mixture is heated enough:
- Aryl iodides can also be obtained from aryl diazonium salts, through treatment with potassium iodide (KI).
- Hydroxyl groups (OH) can be installed on an aryl diazonium salt through heating with water and acid. (we’ve previously seen one example in John Roberts’ work on arynes, which we covered here. )
- Aryl fluorides can be installed through a two step process. The first involves exchanging the counterion (X–) on the aryl diazonium salt with the tetrafluoroborate (BF4–) ion by treating the diazonium salt with HBF4. Then, when heated, fluorine can act as a nucleophile, displacing N2 and releasing BF3 as a byproduct.
- The diazonium salt can also be reduced to C–H, by treating the aryl diazonium salt with hypophosphorous acid (H3PO2).
Not so bad from a single functional group!
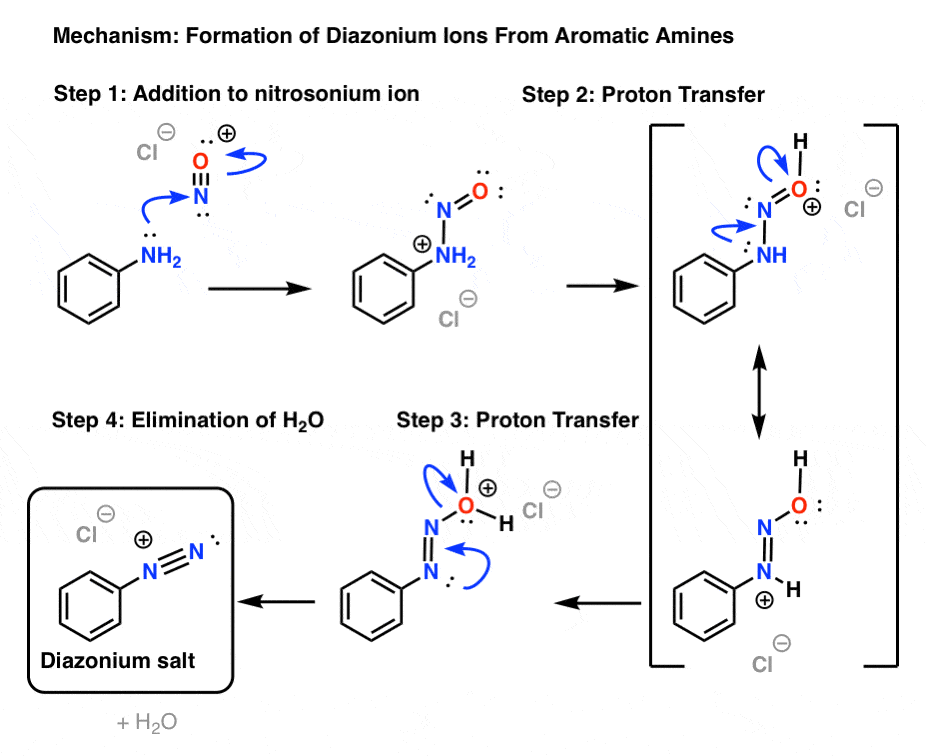
Mechanism: Formation of Diazonium Ions
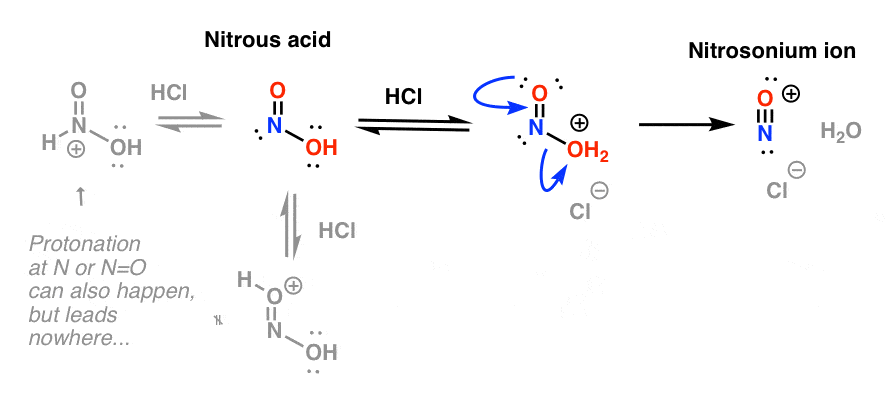
- Formation of nitrosonium ion from HNO2
Now let’s dig into how some of these reactions work.
First, let’s go through formation of the diazonium salt, a process called “diazotization”.
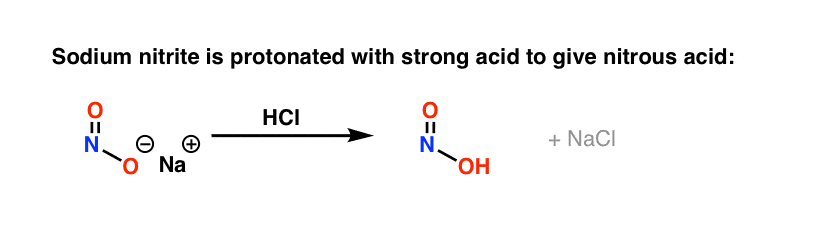
The first key reagent for this process is either sodium nitrite (NaNO2) or nitrous acid (HNO2). Sodium nitrite has the advantage of being an easily handled salt, while HNO2 is a somewhat unstable liquid.
The second key reagent is a strong mineral acid like HCl; if NaNO2 is used, HCl converts it into HNO2.
The key purpose of HCl is to further convert HNO2 into the powerful electrophile NO+, the “nitrosonium ion“, which is the key electrophile in the reaction that forms the diazonium salt.
The nitrosonium ion is formed through protonation of OH and resultant loss of water:
2. Formation of the diazonium ion
The next step is formation of the diazonium ion from the reaction between the amine and the nitrosonium ion, which also requires acid.
How does it work?
The first step is formation of a new N–N bond, which occurs through attack of the nitrosonium ion by the aromatic amine (Step 1). This is followed by two proton transfers from nitrogen to oxygen (Steps 2 and 3) accompanied by reorganization of the pi bonding framework [forming N–N (pi), breaking N–O (pi) ]. The final step is formation of the nitrogen-nitrogen triple bond accompanied by expulsion of water (Step 4).
Being fairly unstable (and potentially explosive), diazonium salts are typically not isolated (it’s relatively safe to handle the tetrafluoroborate salts as solids, but that’s about it). Once formed, they’re usually treated immediately with the appropriate reagent en route to the desired product.
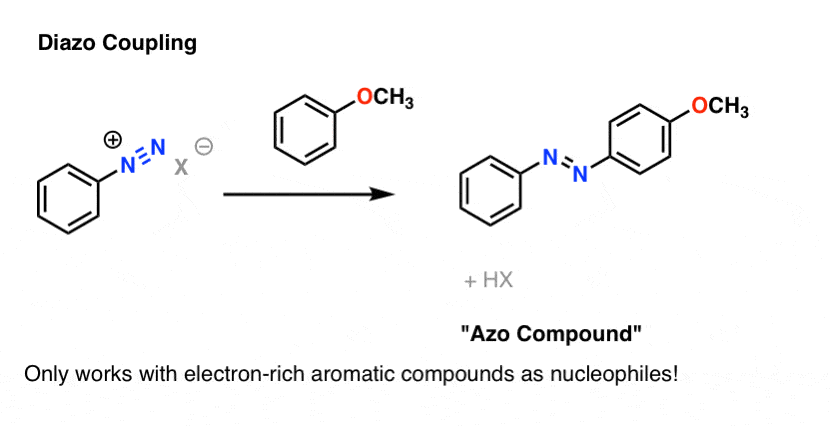
Bonus Reaction: Diazo Coupling
There’s one last reaction of diazonium salts which is worth mentioning. A surprising number of dyes in our daily experience are derivatives of diazobenzene, the essential structure of which is two benzene molecules joined by a nitrogen-nitrogen double bond. See this article on azo dyes, for instance. Yellow, red, and orange are common colors of azo dyes.
Azo dyes are made through the reaction of an electron-rich aromatic partner with a diazonium salt. Only electron-rich aromatic species are good enough nucleophiles to attack diazonium salts.

No comments:
Post a Comment