Factors That Affect Basicity of Amines
From may recall that any factor which makes a molecule’s conjugate base more stable will increase its acidity. [Remember Le Chatelier? If you make the product more stable, you’ll favor the equilibrium going to the right].
What each of the “factors that increase acidity” have in common is that they tend to stabilize negative charge, either through inductive effects, delocalization through resonance, or by bringing the charge closer to the nucleus.
Since acidity and basicity are opposite sides of the same coin, the key factors which affect acidity also affect the basicity of amines. So evaluating basicity involves taking those same concepts but working in the opposite direction.
Generally speaking, the more unstable an electron pair is, the more basic it is. So using the same principles we outlined above, one could increase basicity by removing inductive effects, removing delocalization through resonance, or bringing the charge farther away from the nucleus.
Let’s examine the key factors in turn and apply them to obtain some key trends for the basicity of amines.
Factor #1: Basicity Increases With Increasing Negative Charge On Nitrogen
This is possibly the simplest factor to evaluate. If “basicity” can roughly be translated as “electron-pair instability”, and instability increases with charge density, then basicity should increase with increased negative charge.
A simpler way to put it: the conjugate base of an amine will always be a stronger base than the amine itself.
Compare ammonia, (NH3) with its conjugate base, the amide anion NH2(-). The amide anion is stronger base by far (pKaH of 38, versus pKaH of 9). It can be used to deprotonate terminal alkynes (pKa = 25), for example, whereas ammonia will not.
Continuing this trend, the conjugate base of the amide ion, the amide dianion NH(2-) should be an even stronger base, but it seems to be prohibitively difficult to make. (I’m unaware of a practical application, but would welcome any enlightening comments! ).
Factor #2: “Resonance”, or, Conjugated vs. Non-Conjugated Amines
This relationship between lower charge densities giving rise to lower basicity also applies to lone pairs that can be delocalized into a larger pi system through resonance.
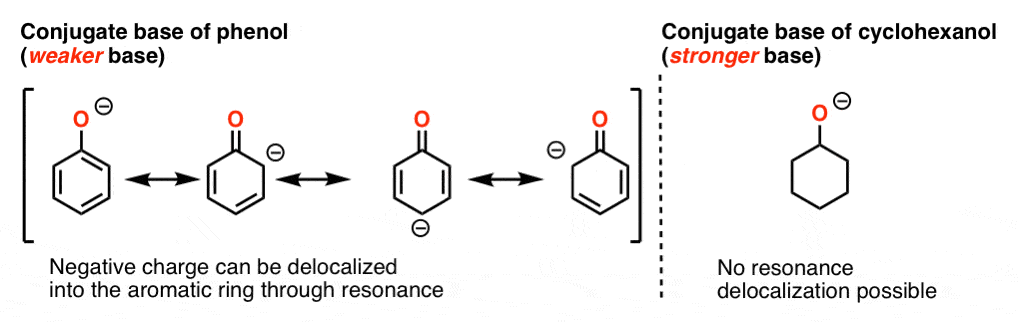
You may recall that phenol (pKa = 10) is a much stronger stronger acid than cyclohexanol (pKa = 16).
Since, “the stronger the acid, the weaker the conjugate base”, this is equivalent to saying that the conjugate base of phenol is a weaker base than the conjugate base of cyclohexanol.
Why? We saw earlier that this is true because the conjugate base of phenol can be stabilized through resonance whereas the conjugate base of cyclohexanol cannot.
The oxygen in phenol is part of a larger “pi system”, and the electron density can be distributed throughout the aromatic ring via resonance. (Remember: lower charge density = more stability).
Let’s apply this to amines.
By analogy, we should also expect that aminobenzene (“aniline”) is a weaker base than cyclohexylamine.
That is indeed the case! The the pKaH of aniline is 4.6, and pKaH of cyclohexylamine is 11.2. (The higher the pKaH, the stronger the base).
The basicity is decreased even further when a second phenyl ring is attached to the nitrogen (pKaH = 0.78).
The bottom line here is that all else being equal, a conjugated amine will be less basic than a non-conjugated amine.
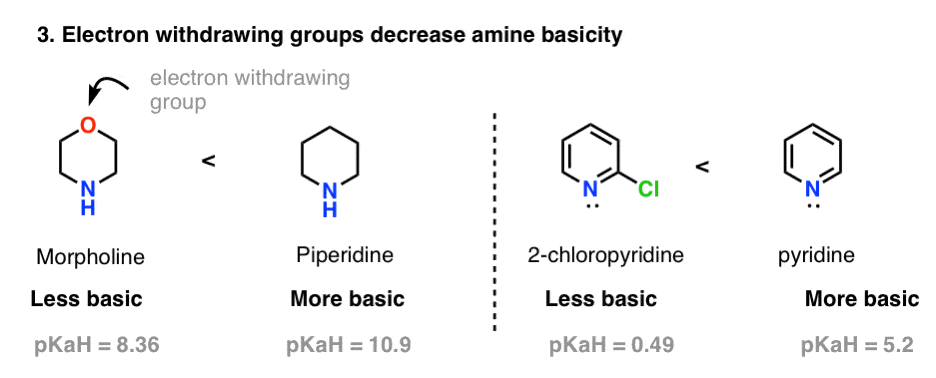
Factor #3. Inductive Effects Decrease Basicity
You may recall that electron withdrawing atoms (e.g. F or Cl) or functional groups (e.g. NO2) tend to increase acidity, by slurping away electron density from the conjugate base. Trifluoroethanol (pKa = 12.5) for example, is far more acidic than ethanol itself (pKa = 16). Lower charge density = more stability = lower basicity.
Hence, we’d expect that electron withdrawing groups on amines should likewise decrease their basicity. And they do! Witness morpholine (pKaH = 8.36) compared to piperidine (pKaH = 11), or 2-chloropyridine (pKaH = 0.49) versus pyridine (pKaH = 5.2).
Factor #4: Pi-Acceptors and Pi-Donors
We’ve seen that resonance tends to decrease basicity (Factor 2) and so do inductive effects (Factor 3).
That said, how do you explain why amides are significantly less basic than amines? Is it resonance? Is it inductive effects? Is it both?

It seems worthwhile to devote a section to how the basicity of nitrogen is affected by its interactions with other functional groups in a pi-system.
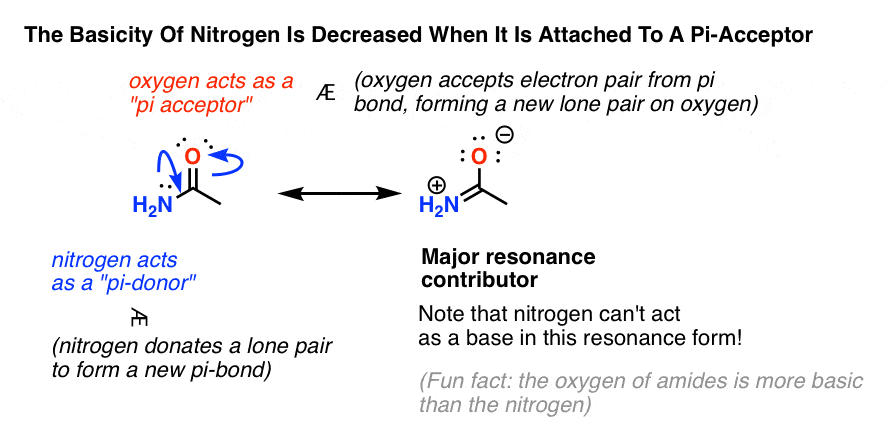
Specifically, the basicity of nitrogen is decreased when it acts as a pi-donor, and the basicity of nitrogen is increased when it acts as a pi-acceptor.
Nitrogen Is Less Basic When It Is A Pi-Donor
Back to our amide example. Why is it less basic?
The first factor is that an electron-withdrawing oxygen is present, which can remove some of the electron density from nitrogen. However, this is outweighed by the fact that there is a significant resonance form where the nitrogen lone pair forms a new pi bond with carbon (we call this, “pi-donation“) resulting in a pair of electrons moving from the C-O pi bond to the oxygen (we call this acting as a “pi acceptor“).
Look at that resonance form on the right. The nitrogen doesn’t have a lone pair anymore, and therefore it cannot act as a base.
Therefore, the basicity of a nitrogen is decreased when attached to a pi-acceptor.
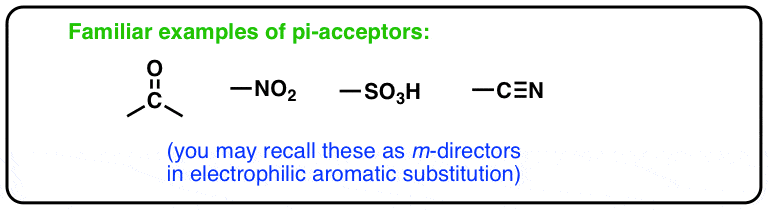
What are pi-acceptors, again? If you’ve covered electrophilic aromatic substitution, these functional groups should seem familiar. You might recognize that “Pi acceptors” all belong in the category of “meta- directors” .
[CF3 is an example of a functional group that is a meta director but not a pi acceptor, since it has no pi bonds]
Nitrogen As A Pi-Acceptor
You might rightly ask if this can work in the opposite direction.
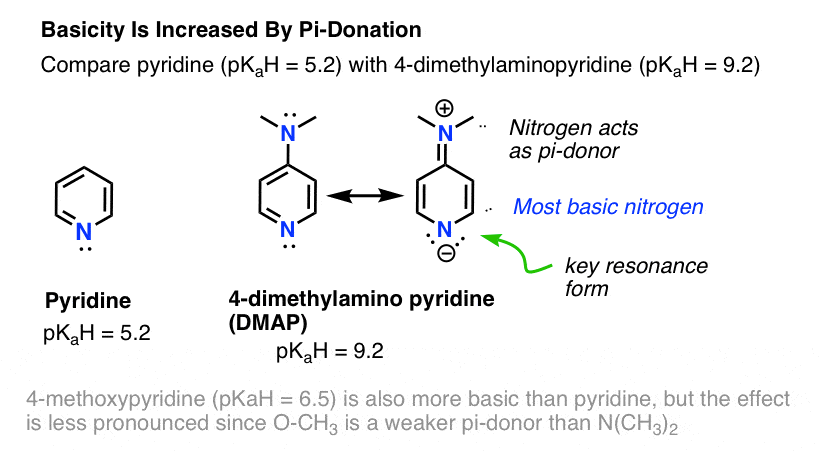
Can the basicity of a nitrogen be increased when it is attached to a pi-donor? Absolutely.
A great comparison is pyridine (pKaH = 5.2) and 4-dimethylamino pyridine (DMAP). Attachment of the strongly pi-d0nating NMe2group to the 4-position results in a 104increase in basicity of the ring nitrogen (pKaH = 9.2). Examining the resonance forms of DMAP is illuminating. In the key resonance form, the nitrogen in the ring bears a negative charge.
The ring nitrogen of DMAP is the most basic nitrogen, not the NMe2! The NMe2 is made less basic by being a pi-donor (see above) but the pyridine nitrogen is made more basic because it is the pi-acceptor here.
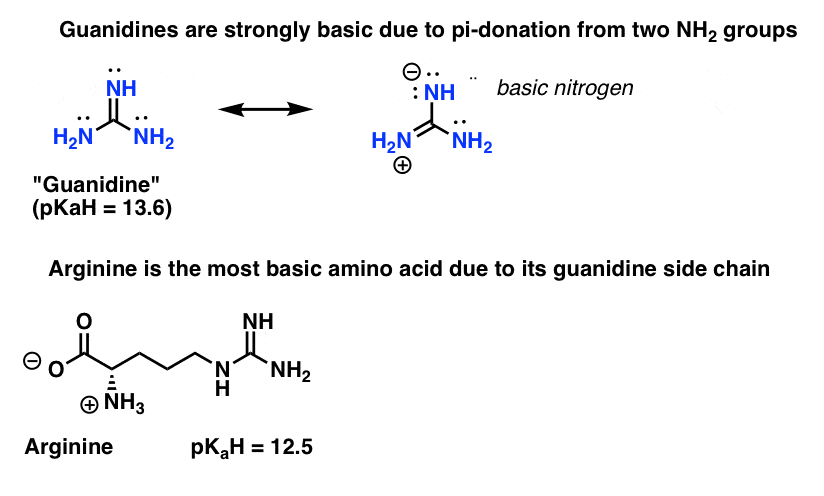
Another example of how basicity of nitrogen can be increased by attachment to pi-donors is found in guanidines. In guanidine there are two pi-donating NH2 groups which can donate electron density to the (pi-accepting) C=NH.
Those of you who have studied some biochemistry might recall that arginine is the most basic of all the 20 essential amino acids (pKaH = 12.5).
Factor #5 . Hybridization
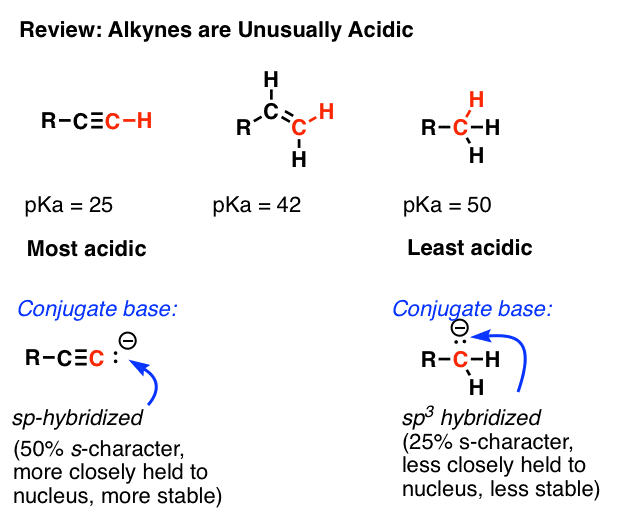
One of the more remarkable acidity trends is that alkynes are unusually acidic (pKa = 25) relative to alkenes (pKa’s around 43) and alkanes (pKa’s >50).
You might recall that our explanation for this effect was that the sp-hybridized orbitals of alkynes bear 50% s-character, and as the 2sorbital is closer to the nucleus than the 2porbitals, the resulting lone pair of the conjugate base “feels” more of the positive charge from the nucleus than would a lone pair in an sp3 hybridized orbital (25% s-character). It’s similar to why a lone pair is more stable on a more electronegative atom like fluorine than on a less electronegative atom like carbon.
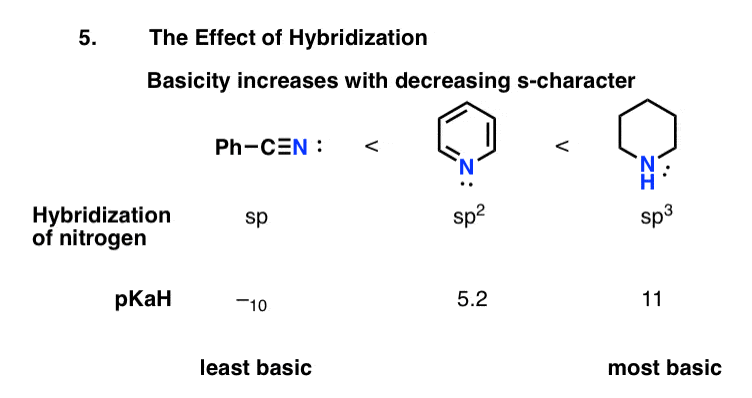
Knowing this, how would you predict the relative basicity of nitriles, pyridine, and piperidine?
By analogy to alkynes, we’d expect the lone pairs in sp-hybridized nitriles to be the most stable and hence the least basic. We’d therefore expect the lone pairs in sp3-hybridized amines to be the least stable and hence the most basic.
This is borne out by pKaH values. The pKaH of benzonitrile (pKaH = –10) indicates that nitriles are very weak bases indeed. We can likewise explain the lower basicity of pyridine (pKaH = 5.2) versus piperidine (pKaH = 11) by the orbital hybridization.
(Not resonance, by the way! The lone pair in pyridine is in the plane of the ring, and thus not in conjugation with the p-orbitals).
Bonus Factor: Aromaticity
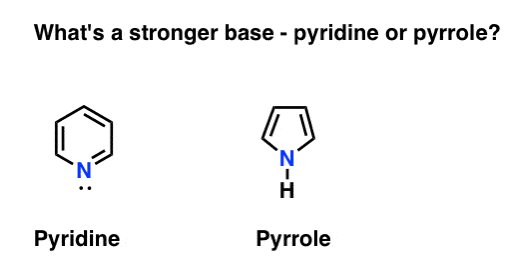
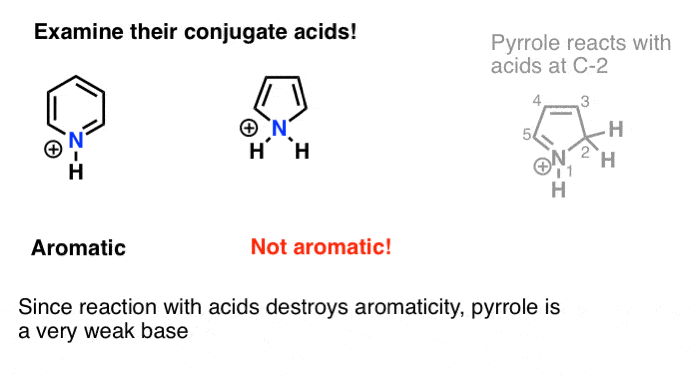
It turns out that the nitrogen in pyrrole is unusually non-basic. In fact, even when subjected to acid, pyrrole reacts at carbon (C-2), and not on the nitrogen. Pyridine [pKaH = 5.2] is far more basic than pyrrole [pKaH about –3.6 ]
Why?
Draw the conjugate acid of pyrrole. Notice anything?
The conjugate acid is not aromatic. Removal of the lone pair on nitrogen through protonation would destroy the conjugation of the lone pair with the other p orbitals of the ring and render the molecule non aromatic.
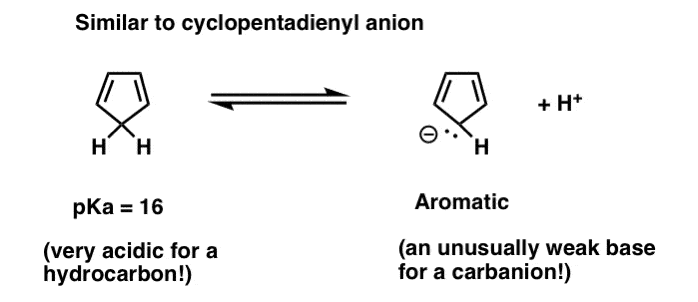
This might ring a distant bell. You might remember that cyclopentadiene is an unusually strong acid for a hydrocarbon. We can restate this as “the conjugate base of cyclopentadiene is unusually weak”.
See the analogy with pyrrole? Protonation of the cyclopentadienyl anion destroys aromaticity.
The bottom line here is to be on the lookout for situations where forming a new N-H bond might disrupt aromaticity. [Note 2]
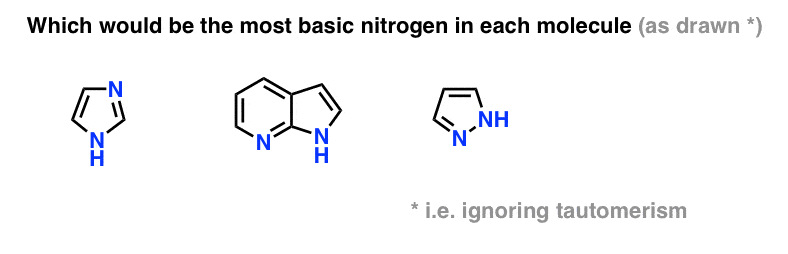
Can you apply the same concept in the question below?
Conclusion
OK, I said there were 5 factors which affected acidity, but I covered six factors here. The reason is that “pi donation” and “pi accepting” behaviour often doesn’t get covered that much in Org 1 (when acidity is introduced) but by the time most students encounter amines, they’ve encountered these concepts in the context of electrophilic aromatic substitution.
The key lesson for today is this: since “the stronger the acid, the weaker the conjugate base” and “the weaker the acid, the stronger the conjugate base”, every factor that affects acidity is likewise a factor that affects basicity.
If you understand the factors that stabilize negative charge (and therefore make an atom less basic), by definition you also understand the factors that destabilize negative charge (and therefore make an atom more basic).
How do we handle situations where multiple factors come into play? We must resort to experimental measurement (pKaH). It’s too difficult to predict trends when more than one variable is being changed at once.
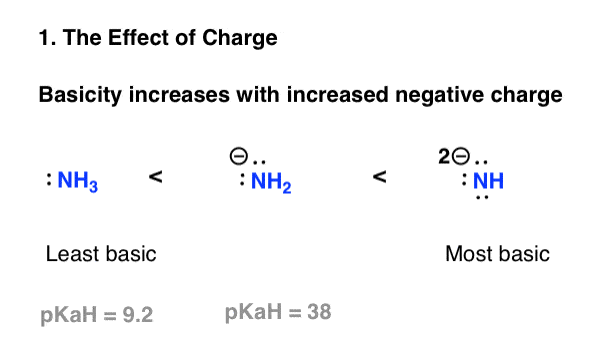
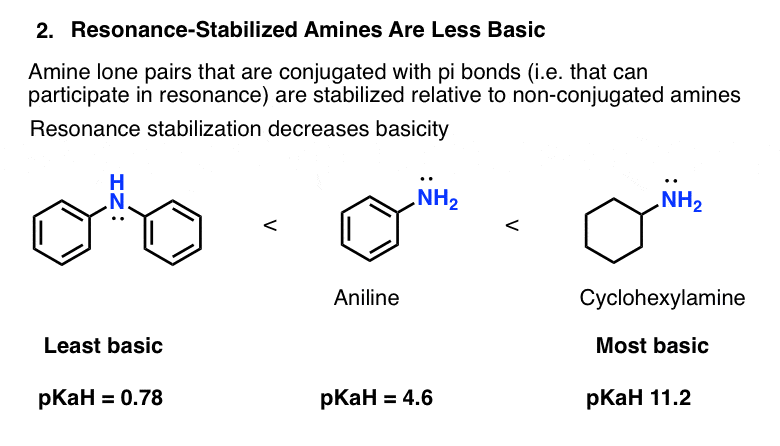

No comments:
Post a Comment